India is finally getting the first consignment of rapid testing kits for COVID-19 from China after delays. According to the sources, the first consignment of 3,00,000 kits is cleared by customs in Guangzhou on Wednesday and headed to Delhi on Thursday. In the next one-two weeks, India is expecting to receive about 2-3 million kits from China.
These kits are the current necessity to control the pandemic. After getting these kits, India will increase its pace in testing COVID-19 patients. Rapid testing kits will play an important role in hotspot areas.
India broadens its horizons to various sources of testing kits starting from Germany, Korea, France and Israel. The health ministry though pointed out that several of these suppliers also get their supplies from Chinese firms.
After the failure of many Chinese kits, the Chinese government prepared the list of the companies whose products undergo the quality test and is eligible for the export. This process may take time said Chinese customs.
Delay in consignment delivery
The government of India placed an order of 7,00,000 lacs kit to Chinese firms. The consignment was expected to get delivered by 8 April. Because of the shortage of cargo planes kits are stuck at ports. G.S.K. Velu Chairman and managing director of Chennai based Trivitron Healthcare said that companies are facing logistic issues.
The delay of the testing kits is slowing down the process of COVID-19 tests. Right now the time is very crucial in India. It is really important to increase the pace of testing in India said the Chairman.
New Approvals
The India based firms have already approved the supply of made in India kits from the Gujrat based Voxtur Bio, Delhi based Vanguard Diagnostics and the government-owned HLL Lifecare. Because of the delay of china based test kits, on April India’s Apex Medical research body, ICMR approves the use of these kits in the areas with a high number of COVID-19 cases and which are hotspots.
The ICMR approved the new sources of testing kit manufacturers from Korea, Germany and France, most of them are Chinese firms. On April 8 list of new manufacturers approved. Around 33 suppliers got approval out of which 31 are Chinese firms and rest two are from Korea and Israel.
Rapid test kits importance
A rapid test kit shows if a person once infected by COVID-19 has developed immunity in it. The human bodies develop two types of antibodies- immunoglobulin M (IgM) and immunoglobulin G (IgG). They remain in the body for a longer period i.e a month or a year.
The IgM arrives within four to seven days of infection and IgG arrives after the recovery from the infection. When the person is IgG positive it means the patient is exposed to the infection and has developed the immunity in the response of that infection.
Rapid testing COVID-19 kits
These tests are relatively cheap than the RT-PCR test which is currently the standard COVID-19 of the country. RT-PCR test costs around Rs 4,500 done in a private lab whereas rapid test kit will cost around Rs 300.
On April 16, the total number of global cases has surpassed 2 million, including more than 138,000 deaths and 525,000 patients recovered.
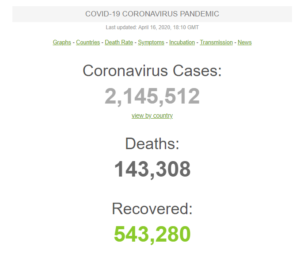
Global Count of Coronavirus Cases
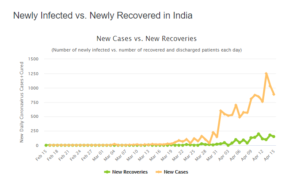
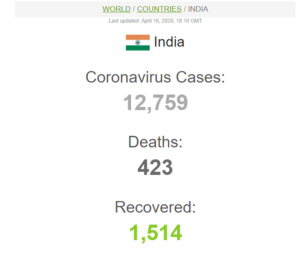
On April 16, the total number of cases in India reached to 12,759 including 423 deaths and 1,514 recovered. The coronavirus is slowly increasing its pace in India. The testing kits will be a boon to the hotspots of COVID-19.
Coronavirus cases in India